The Chemical Formula for Epsom Salt is (MgSO4.7H2O) so it contains no chlorine or sodium or no “table salt”. Even though it readily dissolves in cold water The Magnesium molecule and the Sulfur molecule are bonded together with four molecules of oxygen that are tied together with 7 molecules of water.
IF you get a “Soil Test Analysis” report it will also list how much Sodium or “salt” left over’s are in your soil in the area that you test from.
In order for a plant to absorb and actually use either the Magnesium or the Sulfur as a single molecule then I would expect that this chemical bond has to be broken either in the soil by microbes or within the plant cells themselves or the “Magnesium sulfate” molecule will/could be useless to the plant.
I prefer to mix in the micronutrients into water and then apply this to the soils with deep watering. Magnesium spread on top of the soils might not get broken down into a plant useable form until it is too late for this growing season. Remember that this Magnesium also needs to get all the way down to the root zone where the majority of the daffodil bulb roots will be pulling up water and nutrients.
When to apply fertilizers and nutrients?
I never really under stand why so many printed articles say to wait until late in the season to fertilize bulbs! Think of a daffodil as a new born human. Do we wait to give newborns a complete vitamin packed diet when they become teenagers and are mostly grown or do we make sure every mineral and vitamin is in their baby formula:-))
Again I am NOT SURE why we spend hundreds of $ just on the bulbs and hundreds of hours of time devoted to the bulbs but we can’t spend 1/2 the cost of a single sack of fertilizer on a soil test to see just what our soils are lacking where we have invested (planted) so much of our treasure!
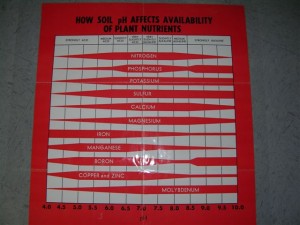
If you look at the photo of the PH chart you will see that no matter how much Magnesium that you have in your soil that IF you have Acid soils of say a PH of 5.0 then VERY little of the Magnesium will remain “readily available” to any plant roots. Ideally your daffodils and most vegetable plants would benefit from a soil PH of 6.5 as nearly all of essential plant nutrients and micro nutrients are readily available at that PH level. Keith Kridler Mt. Pleasant, Texas

The chemical bond on magnesium sulphate is ionic, which means that in watery solution MgSO4 exists as Mg++ ions and SO4– ions. The heptahydrate crystalline structure found in the “dry” state of the fertilizer breaks down in liquid water. (Molecules of liquid water are attracted by the charges on the positive and negative ions, and surround the ions, but not as crystals). Plants can readily absorb these ions when they absorb water through their roots.
—-